Abstract
Introduction: Axicabtagene ciloleucel (axicel) and tisagenlecleucel (tisacel) chimeric antigen receptor (CAR) T-cells targeting CD19 were firstly approved for the treatment of relapsed/refractory (R/R) large B-cell lymphomas failing ≥2 lines of chemotherapy. The estimated 2-year progression-free survival approaches 40% in pivotal trials and real-life studies suggesting that a large proportion of patients (pts) is not cured. The therapeutic approach for pts who are refractory to or relapse after CAR T-cell has not yet been established.
Methods: This is a retrospective study enrolling 51 consecutive pts failing axicel (n=17) or tisacel (n=34) which were treated at Hematological Centers that had access to early phase clinical trials using Glofitamab (NP30179) or Loncastuximab tesirine (ADCT-402-103). According to the tumor and clinical characteristics, pts were treated with novel drugs, standard therapies, or supportive care. Overall survival was estimated from the day of CAR T-cells infusion to the last follow-up [pts receiving allogeneic transplantation (allo-SCT) were censored at the date of transplantation]. For pts receiving a treatment, further analysis of OS and PFS was performed by considering the time from the beginning of salvage to the last follow-up
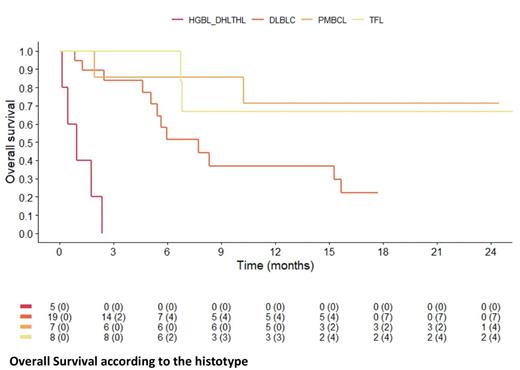
Results: From 02/2019 to 02/2022, 51 pts [29 DLBCL (57%), 7 high-grade B-cell lymphoma (HGBCL, 14%), 8 transformed follicular lymphomas (tFL, 16%), and 7 primary mediastinal B-Cell lymphoma (PMBCL, 13%)] were included in this retrospective analysis. At time of CAR T-cell infusion, 36 (71%) of the patients presented with primary refractory disease and 17 (33%) had intermediate-high and high IPI. Twenty of 51 pts (39%) experienced a transient response to CAR T-cells whereas 31 (61%) never responded. Failure after CAR T-cells occurred at a median of 49 days (IQR: 31-92.50) with 9 patients (18%) experiencing an early progression (≤30 days). Twenty-nine (57%) were biopsied, among whom evidence of CD19 and CD20 loss was in 12 (41%) and 8 (28%) cases, respectively. Twenty-two (43%) pts were enrolled in a clinical trial [n=18 Glofitamab (Glofit), n=4 loncastuximab+ibrutinib]. The remaining 29 pts received either salvage therapies (n=17) [lenalidomide (LEN, n= 4), checkpoint inhibitors (CKI, n=4), ibrutinib (n=2), chemotherapy (n=6), radiotherapy (n=1)] or supportive care (n=12). No significant differences in pts characteristics were observed between pts enrolled or not in a clinical trial. Immunotherapy (Glofit, LEN, CKI) versus no immunotherapy was associated to a trend to higher CR rate (45% versus 15%, p=0,06). The overall response and the CR rate were 68% and 38% in pts receiving Glofit [with an improved CR rate (57%) in transient responders to CAR T-cells]. Nine patients received alloSCT as a consolidation of clinical remission (6 after immunotherapy and only 3 following standard treatments). At a median follow-up of 12 months, 19 pts (37%) are alive and 32 died from disease progression (n=30) or toxicity (n=2) resulting in 1-year OS for all pts of 35% (95%CI, 23%-53%). Seven out of 9 allografted pts are alive in remission. A better outcome in terms of OS was observed for PMBCL and tFL vs others histologies (p<0.0001), for transient responders to CAR T-cells (63% vs 17%, p<0.0010) and for those with IPI 0-1 vs 3-5 (54% vs 24%, p<0,009). The estimated 1-year OS was 44% (95%CI,30%-66%) for those undergoing a salvage therapy, with a trend for a better prognosis among those treated with immunotherapy (1-year OS: 52% vs 30%, p=0.0807), respectively. In multivariate analyses for OS for all pts, factors adversely affecting the prognosis were absence of response to CAR T-cells [HR 3.08; p=0.0109] and diagnosis other than PMBCL and t-FL [HR 4,54 p=0.0069].
Conclusions: Immunotherapy with Glofit, LEN or CKI may provide significant clinical responses, particularly in transient responders to CAR T-cells. Transient response to CAR T-cells is likely to be associated with an anti-tumoral microenvironment resulting in a better response to immunotherapy post-CAR T-cells. The histotype emerges as relevant factor in define prognosis in pts relapsing after CAR T-cells.
Disclosures
Chiappella:Ideogen: Other: advisory board; SecuraBIO: Other: advisory board; Novartis: Other: lecture fee; Incyte: Other: lecture fee; Astrazeneca: Other: lecture fee; Takeda: Other: lecture fee, advisory board; Roche: Other: lecture fee, advisory board; Janssen-Cilag: Other: lecture fee, advisory board; Gilead Sciences: Other: lecture fee, advisory board; Clinigen: Other: lecture fee, advisory board; Celgene-Bristol Myers Squibb: Other: lecture fee, advisory board. Santoro:Merck Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Abb-vie: Speakers Bureau; Amgen: Speakers Bureau; Sandoz: Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli-Lilly: Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Sanofi: Consultancy; Incyte: Consultancy; Takeda: Speakers Bureau; Roche: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Carlo-Stella:Karyopharm Therapeutics: Other: Consultancy/Advisory; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Novartis: Honoraria; Takeda: Honoraria; AstraZeneca: Honoraria; Incyte: Honoraria; Janssen Oncology: Honoraria; Merck Sharp & Dohme: Honoraria; Bristol Myers Squibb: Honoraria; Sanofi: Other: Consultancy/Advisory, Research Funding; Roche: Other: Consultancy/Advisory, Research Funding; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding; Scenic Biotech: Other: Consultancy/Advisory. Corradini:takeda: Honoraria; janssen: Honoraria; incyte: Honoraria; gilead: Honoraria; celgene: Honoraria; amgen: Honoraria; abbvie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.